




The #1 used GPI in the U.S.
20+ years on the market since approval of first indication in 1998.
Over 20 years of clinical research.
Used in over 400,000 patients since 2013.
The following profiles are not actual patients. Individual examination is required to determine the best course of treatment.
Please see Important Safety Information and Full Prescribing Information.
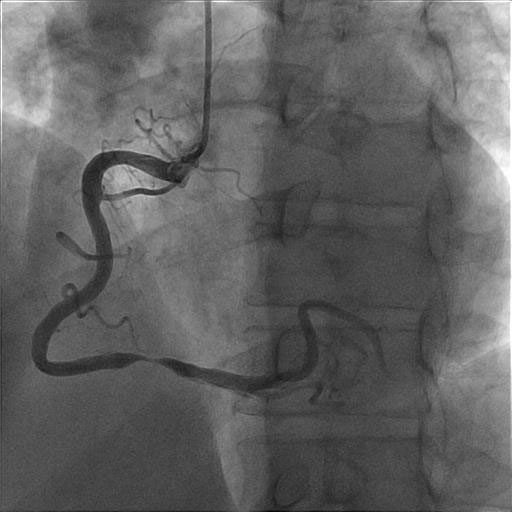

Symptoms
Medical History
Medications
Physical Examination and Lab Results
Action:

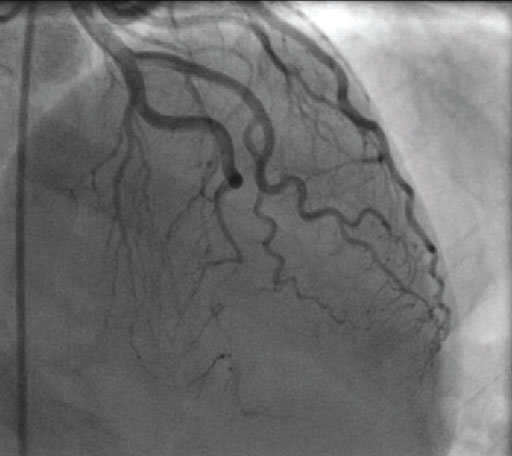
Symptoms
Medical History
Home Medications
Concomitant use of Aggrastat with fibrinolytics, anticoagulants and antiplatelet drugs increases the risk of bleeding.
Physical Examination and Lab Results
Action
For renally impairment patients with creatinine clearance ≤60 mL/min a dose adjustment for Aggrastat is required. Renally impaired patients should receive a single bolus (25 mcg/kg within 5 minutes), and a maintenance infusion of 0.075 mcg/kg/min for up to 18 hours.


Symptoms
Medical History
Aggrastat is contraindicated in patients with active internal bleeding, or history of bleeding diathesis, major surgical procedure or severe physical trauma within the previous month.
Home Medications
Concomitant use of Aggrastat with fibrinolytics, anticoagulants and antiplatelet drugs increases the risk of bleeding.
Physical Examination and Lab Results
Action


Symptoms
Medical History
Home Medications
Concomitant use of Aggrastat with fibrinolytics, anticoagulants and antiplatelet drugs increases the risk of bleeding.
Physical Examination and Lab Results
Action

Aggrastat is available through normal distribution at your wholesaler. For immediate supply, Aggrastat is available as a drop shipment via Express Overnight Delivery. It is important to provide your wholesaler with updated demand forecasts for Aggrastat.
This tool is a resource you can use to calculate a patient’s Creatinine Clearance
Using the patients gender, their actual body weight (in kilograms),
and their Serum Creatinine levels (in mg/dL).

Aggrastat® is indicated to reduce the rate of thrombotic cardiovascular events (combined endpoint of death, myocardial infarction, or refractory ischemia/repeat cardiac procedure) in patients with non-ST elevation acute coronary syndrome (NSTE-ACS).
For additional information, refer to the full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.FDA.gov/medwatch
or call 1-800-FDA-1088.
* This website is intended for U.S. healthcare professionals only.
